“Undisclosed problem
In documents obtained through the pretrial discovery process (see supplementary data on bmj.com), prosecutors suing the Irish Minister of Health, Health Service Executive, Health Products Regulatory Authority, and GSK have found a string of GSK postmarketing safety reports that show a striking
difference in the number and frequency of adverse events reported for three GSK pandemic vaccines approved and used across the world: Pandemrix, Arepanrix, a similar H1N1 vaccine that also contained AS03 adjuvant, and an H1N1 vaccine without adjuvant (no brand name is given).
The BMJ learnt of the reports from my colleague Tom Jefferson, a medically trained epidemiologist who was hired as an expert witness by the solicitors representing Aoife Bennett, an Irish woman who developed narcolepsy after vaccination with Pandemrix in 2009. Jefferson took on the case in 2015, and last year the lawyers received a copy of the GSK safety reports that had been emailed within the company and to at least one regulator (Ireland). Adverse event tables embedded in nine reports spanning the four months between December 2009 and March 2010 offer a glimpse into the vaccines’ safety profiles.
‘When I saw those tables, I just fell off the chair. A consumer can figure out what’s going on here,’ Jefferson told me (table 1). Jefferson immediately calculated the adverse event rates for each vaccine, which showed large differences between Pandemrix and Arepanrix. Any real differences between the
vaccines would be especially alarming because Pandemrix and Arepanrix are, broadly speaking, the same vaccine manufactured in different facilities and used in different countries. Divergent rates of adverse events might implicate a manufacturing problem.
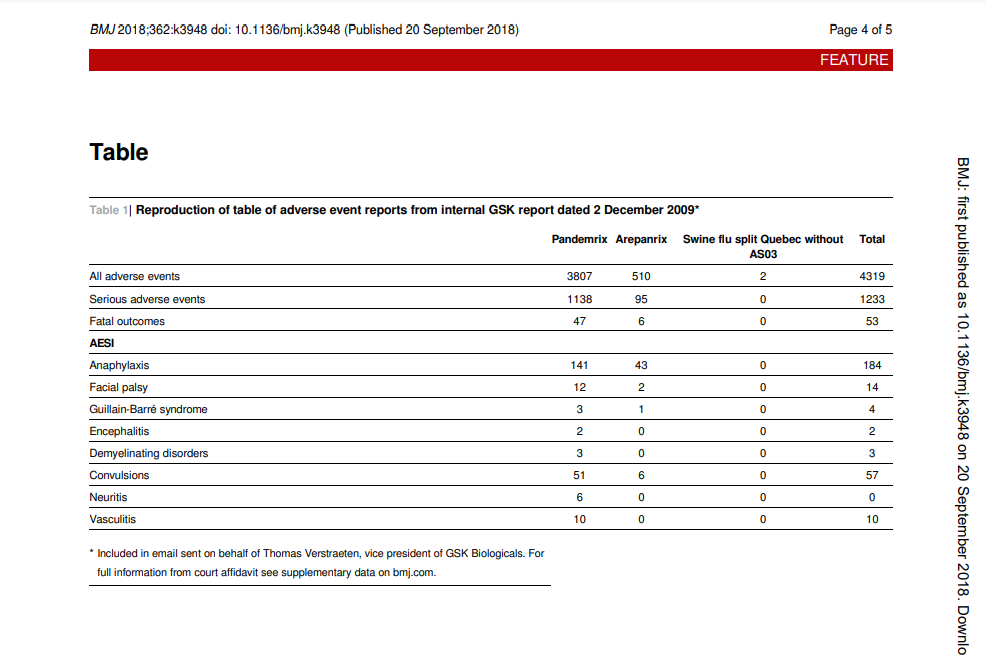
‘The odds ratios, the point estimates, are all high. And some of them are significantly high—5.39 [95% confidence interval 3.70 to 7.85] for deaths [for Pandemrix v the other vaccines],’ Jefferson said. ‘The thing that struck me was not just that the odds ratios were high, but the fact that nobody had tabulated and analysed them,’ he said, pointing out that the GSK reports provided numerator and denominator data sufficient to calculate the odds ratios but did not actually contain those calculations.
The BMJ conducted its own analysis of the adverse events, most of which seem to have been reported spontaneously to GSK (figs 1 and 2). For a range of concerning adverse events, reports were coming in for Pandemrix at a consistently higher rate than for the other two GSK pandemic vaccines–four times the rate
of facial palsy, eight times the rate of serious adverse events, nine times the rate of convulsions. Overall, Pandemrix had, proportionally, five times more adverse events reported than Arepanrix and the unadjuvanted vaccine. And the raw numbers of adverse events were not small.
Although it is often said that perhaps only up to 10% of adverse events are reported to national reporting systems, by late November, GSK had received 1138 serious adverse event reports for Pandemrix—a rate of 76 per million doses administered. By mid-December, there had been 3280 serious adverse event
reports (68/million doses). The last report seen by The BMJ, dated 31 March 2010, shows 5069 serious adverse events for Pandemrix (72/million doses), seven times the rate for Arepanrix and the unadjuvanted vaccine combined.
The data are insufficient to draw conclusions about cause and effect, but for Gillian O’Connor, the solicitor representing Bennett, they raise serious questions about transparency. The disparity, she wrote in an affidavit filed in court, was ‘of such striking difference that any person contemplating taking the Pandemrix vaccine would be likely, if in receipt of this information, not to choose to have the Pandemrix vaccination.’
Alarm bells that never rang
But neither GSK nor the health authorities seem to have made the information public—nor is it clear that the disparity was investigated. This is in contrast to the reaction to narcolepsy, which quickly made news headlines and was the subject of a GSK press release and investigation17 in a matter of weeks after
the first reports from Sweden and Finland.
In many of the GSK reports, the company briefly mentions having conducted ‘safety reviews’—for example, with respect to anaphylaxis, facial palsy, and Guillain-Barré syndrome. The BMJ asked GSK for a copy of those reviews but it did not provide them. In a statement, GSK wrote: ‘the introduction of
Pandemrix, GSK continuously evaluated all available safety data and shared the data with the EMA and other regulatory authorities where the vaccine was licensed so that the authorities could conduct their own independent assessments. EMA made weekly summaries of the data provided by GSK and other
manufacturers publicly accessible and they remain accessible through the EMA’s website.’
The BMJ asked GSK whether it ever undertook any investigations to understand the discrepancy in adverse event reporting between Pandemrix and Arepanrix, whether it notified healthcare providers about the discrepancies, whether it considered pulling Pandemrix from the market, or considered recommending Arepanrix or another company’s vaccine. But GSK declined to answer these and all of The BMJ’s questions, citing ongoing litigation.
The BMJ asked the UK Department of Health why it recommended Pandemrix over Baxter’s Celvapan, but the department also declined to comment, calling the question ‘quite technical’ and suggesting we submit a freedom of information request for an answer. In December 2009 demonstrators in Scotland took to the streets to challenge the government’s swine flu vaccine campaign arguing it was out of step with the mild pandemic.”

You can read here.

